Sep 14, 2024
Science News
Abbott Relaunches Deep Brain Stimulation Trial for Depression
Abbott has announced a new clinical trial for its Infinity Deep Brain Stimulation (DBS) system, aimed at treating treatment-resistant depression. This follows a similar trial from 9 years ago, which was halted after inconclusive results. The new trial will involve patients who have not responded to at least four different antidepressant therapies.
How the Infinity DBS System Works
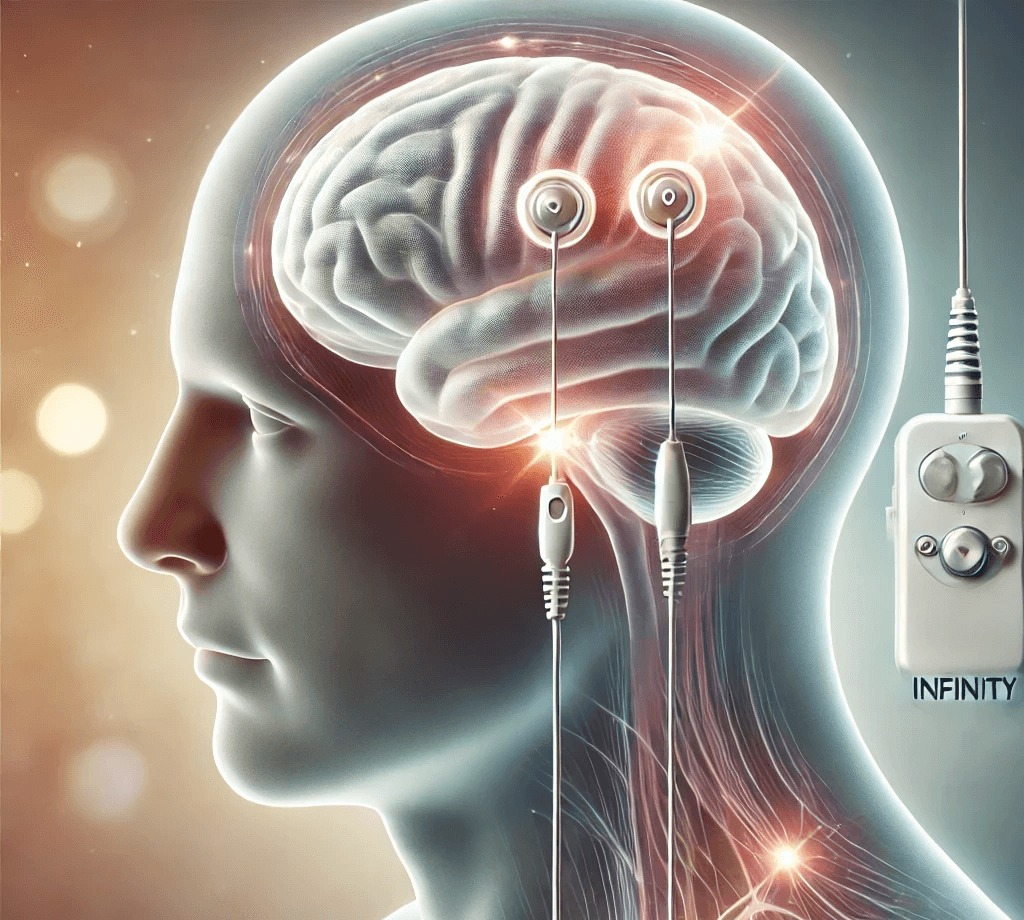
DBS works by delivering electrical signals to specific areas of the brain through electrodes. In this case, the device targets the subcallosal cingulate, a region associated with mood regulation. It has already been FDA-approved for conditions like Parkinson’s and essential tremor, but this trial will focus on its potential for treating depression.
Why Abbott is Restarting the Study After 9 Years
Abbott’s original trial did not show a significant improvement in depression symptoms, but advancements in technology and study design have led to this new effort. The company now has tools like diffusion tensor imaging to more accurately target brain regions, and the trial will measure outcomes over a longer period—12 months instead of 6.
Technological Advances in Treating Depression
The improvements in imaging technology and study design are expected to increase the chances of success. With more precise targeting and extended observation, researchers hope to see more meaningful results in reducing symptoms of treatment-resistant depression.
What to Expect from the New Study
The study will recruit 100 participants, half of whom will receive the active treatment while the other half receive a placebo. Researchers will compare depression symptom severity between the two groups over one year. The trial is expected to conclude in 2029, with hopes of offering a new solution for the many patients who struggle with depression that does not respond to existing treatments
Read more here
To be always in the loop about the latest healthcare trends, follow 360/Health